Smartrick Pick's Present: Bels Bombs: We're Talking Testes, Traders. | LPCN |
An in-depth look at Lipocine and its upcoming FDA decision on TLANDO from its newest contributor The Bombastic Bels

Disclaimer: As of publishing, the writer has a position in LPCN. I am not a financial advisor, this should not be taken as advice. All information used is publicly available. Make your own decisions.
LPCN | FDA Play Edition - We’re Talking Testes, Traders by The Bombastic Bels
Introduction:
Bels here, excited to partner with the legendary Smartrick and drop a spicy Bels Bomb. I am an equal parts technical and fundamental swing trader that seeks to visualize and leverage catalyst based hype in this kangaroo market we find ourselves in. I enjoy digging deep into FDA plays and simplifying all the noise for those who don’t know a PDUFA from a PDF file.
Overview:
Company: Lipocine
Ticker: $LPCN
Lipocine is a clinical-stage biopharmaceutical company focused on metabolic and endocrine disorders that are currently awaiting an FDA decision on TLANDO, an oral testosterone replacement therapy designed to help with Low-T and hypogonadism.
This stock caught my eye because of the upcoming FDA decision, the predicted explosive growth of the low-t market, and the overall technicals of how this stock is trading right now.
Table of Contents:
Charts
What is Lipocine’s TLANDO?
Potential Competition
Lipocine/Clarus Lawsuit
History of TLANDO and FDA
TRT Market Size
Earnings
Risks
Next In The Pipeline For Lipocine
Main Upcoming Catalyst: FDA PDUFA Decision Date on TLANDO - Set For August 28th
Current PPS: $2.14 at close 08/14, gapped up to $2.21 in Afterhours
The Charts
1 Year 1 Day Chart

Bels Comment:
As you can see, LPCN has been on a steady upswing since it crashed in November of 2019. The November 11th FDA denial and earnings occurred right around the bottoming out of share price.
5 Day 5 Minute

Bels Comment:
Here we see the PPS heading back toward the recent high of $2.33 as anticipation grows over the upcoming FDA decision. Note that after close LPCN broke the upper band of VWAP toward the end of 08/14.
ELI5
Stonks go up and may continue to go up until the FDA decision on August 28th.
Indicators I use
ADX/DMI
I use ADX/DMI to better understand long term trends and see if there is confidence in the data that a positive or negative trend may continue.
Pro-tip using ADX/DMI: Longer look backs tend to offer stronger signals.
Using ADX DMI Indicators on the 5 Day chart, we can see the green positive DMI indicator on a sharp upswing with the ADX (the white line) confidence score (24) beginning to trend upward. This suggests that there is confidence the stock is in a positive trend - something I always look for in a swing play.

Backing it up a bit more to the 10 Day chart we see a clearer picture of a rising trend, both in positivity and confidence.

Final thoughts on the price action:
In 2019 the stock price jumped to as high as $3.4, early indications point to positive trend developing. It has been on a fairly steep incline toward the end of the week ending above VWAP at $2.21. I am bullish on LPCN continuing to fill the gap up to FDA decision. From then on of course the decision will have a large impact on price action from then on.
ELI5
Data suggests confident upward trends for the price per share in the near future



What is TLANDO?
TLANDO is an oral testosterone replacement therapy designed to help with Low-T (and other things)!
Is Low-T a problem? Yes. The American Urology Association reports that low testosterone affects about 2% of all men. The risk only increasing with age.
TLANDO is here to help the over-one-million men dealing with low-t as well as those with Hypogonadism.
What the hell is hypogonadism?
Male hypogonadism, also known as testosterone deficiency, is a failure of the testes to produce the male sex hormone testosterone, sperm, or both.
Is hypogonadism a problem? YES. Hypogonadism affects an estimated 4 to5 million men in the United States
Enter TLANDO. TLANDO is an oral therapy that contains a little something called Testosterone Undecanoate.
What is Testosterone Undecanoate?
Testosterone undecanoate is a long-acting man-made version of testosterone, the natural male sexual hormone.
What Makes $LPCN’s TLANDO Special?
Other TRT Therapies exist, why is TLANDO any different from some of its competitors?
Because it’s ORAL.
Ask a TRT expert, they’ll all tell you the issues with current non-oral TRT options.
Here are a few:
Black Box Warning
Secondary exposure to testosterone
Pulmonary oil micro embolism (POME) and anaphylaxis shock… yikes
Inconvenient application or painful injection
More than 50% of patients need a dosage adjustment
Burdensome for patients due to multiple doctor visits
ELI5
TLANDO is great in how it could potentially deal with hormone issues, but its true value is that it is oral instead of injectable.

What Oral Testosterone Therapies Are Currently Available In The US?
We’ve all seen the Roman Testosterone ads, do they work? Let’s take a look into what you’re actually buying:
Roman Testosterone support is a combination of Vitamin D3, zinc, magnesium, copper, ashwagandha, and maca. Translation: A mix of one vitamin, 2 minerals, a root, and a shrub… I’ll let you decide for yourself if that’s the holy grail of hormone therapies.
In other words, vitamins, and minerals...
Okay, so are there any Oral Testosterone DRUGS available?
Yes and no. Clarus and Lipocine are engaged in a patent dispute over how similar their oral testosterone therapies are. There is a trial date set for February of 2021 BUT thus far the courts have ruled in favor of Lipocine, which is GOOD news.
In other parts of the world, Australia specifically, a product called Adriol Testocaps is available for use using, you guessed it, Testosterone Undecanoate.
Lipocine vs Clarus
The Skinny On-Court Proceedings
Both companies have been in a dispute since at least 2016 when Clarus said Lipocine's male testosterone replacement therapy Tlando, or LPCN 1021, infringed on its patent number 8,828,428. However, the U.S. Patent Trial and Appeal Board made a ruling that effectively canceled Clarus' patent.
In April 2019, Lipocine fired back with its own lawsuit against Clarus, seeking to prevent the Northbrook, Ill.-based company from marketing testosterone deficiency treatment Jatenzo, which Lipocine said infringed on six of its U.S. patents.
Read that again.
If the higher courts agree with the decisions of the lower courts and if they get approval for TLANDO, then there is potential for Lipocine to be the major FDA approved oral testosterone therapy available in the United States.
Has TLANDO Been Up For FDA Approval Before and Been Rejected?
Yes, 3 times. This is their 4th attempt.
Lipocrine received a Complete Response Letter (CRL) of denial in June of 2016
The CRL identified deficiencies related to the dosing algorithm for the label. Specifically, the proposed titration scheme for clinical practice was significantly different from the titration scheme used in the Phase 3 trial leading to discordance in titration decisions between the Phase 3 trial and real-world clinical practice.
CRL received May 2018
Citing 4 deficiencies
The CRL identified four deficiencies which include the following: determining the extent, if any, of ex vivo conversion of testosterone undecanoate ("TU") to testosterone ("T") in serum blood collection tubes to confirm the reliability of T data; obtaining definitive evidence pre-approval via an ambulatory blood pressure monitoring study as to whether TLANDO causes a clinically meaningful increase in blood pressure in hypogonadal men; verifying the reliability of Cmax data and providing justification for non-applicability of the agreed-upon and prespecified Cmax secondary endpoints for TLANDO; and, determining the appropriate stopping criteria that can reproducibly and accurately identify those patients who should discontinue use of TLANDO.
CRL received November 2019
Citing 1 deficiency
The CRL identified one deficiency stating the efficacy trial did not meet the three secondary endpoints for maximal testosterone concentrations ("Cmax").
Information needs to be re-analyzed and resubmitted to ensure efficacy and data validity
Things look better for Lipocine if data can be verified via a reanalysis of findings as stated in 2019’s CRL.
Why I am bullish on Lipocine being granted approval
Lipocine was given 6 months to address a single deficiency, they were able to address 3 deficiencies between FDA attempts 2 and 3.
ELI5
They have proven the ability to address many of the initial problems and likely only need to prove efficacy by re-analyzing findings and resubmitting.
Why Should I Care About Lipocine?
Because of the money. Global Testosterone Replacement Therapy Market is expected to exceed more than US$ 1.0 billion by 2024 at a CAGR of 4% in the given forecast period. Testosterone is responsible for the improvement of male sexual characteristics and this hormone formed by the testicles.


If approved, Lipocine’s Tlando has the potential to have a big slice of a pie that is getting bigger every single year.
Who Are The Current Players In Testosterone Replacement Therapy?
According to Marketdataforecast.com, the big players heading into the next few years are:
Pfizer,
Bayer,
Eli Lilly,
Others - BIG Pharma names.
The Financials
Recent Q3 Financial Highlights
Lipocine reported a net loss of $6.4 million, or ($0.13) per diluted share, for the quarter ended June 30, 2020 - Up .01 year over year for the quarter that ended June 30, 2019
Research and development expenses were $2.3 million for the quarter ended June 30, 2020
As of June 30, 2020, Lipocine had $18 million of unrestricted cash, cash equivalents and marketable investment securities compared to $14.1 million at December 31, 2019
The Company had $5.0 million of restricted cash, which is required to be maintained as cash collateral under the SVB Loan and Security Agreement until or in the event that TLANDO is approved by the FDA.
Recent Insider Movement
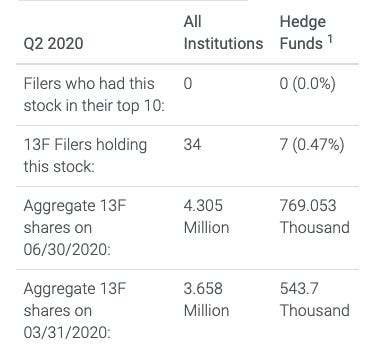

ELI5
Quite a few more institutions created or added to their position than reduced or closed their positions. For me, this is simple swim with the whales logic.
Two Biggest Risks Ahead of FDA PDUFA Decision on August 28th
Offering
This will always be a risk. Less than 20 million for cash on hand could be a cause for concern, but LPCN may be better off to wait until the FDA decision to raise more capital IF that is something they decide to do
Cash on hand increased, quarter over quarter, something I view to be as encouraging
ELI5
Penny stock companies are often shit and need to raise money. When they make an offering they are adding more shares, at often at a lower price. These two factors can make the share price drop quickly. Companies can do this when they think interest in a company is high.
FDA Denial
Of course, this is the biggest risk associated with holding through a decision. Make the choice that is best for you
ELI5
Yikes. FDA denials are baaaaad for the price per share.
Future Pipeline for Lipocene

The Bels Bombs’ Bottom Line:
LPCN makes sense for me for three reasons:
Love the chart and its consistent upward trend
Huge catalyst ahead that could change the game for low-t and hypogonadism within the United States
Solid company with decent financials and many drugs in the pipeline
It’s not a matter of if the low-t/hypogonadism market blows up, it’s when. If things shake outright, $LPCN could be the future of dealing with a big problem.



